- Prologue
- Matter classification
- Properties of a Substance
- SI system of measurement
- 7 Basic units in SI measurement
- Definition of SI units
- Difference between Mass and weight
- Why Pt-IR cylinder for weight standardization?
- Temperature
- Five Laws of Chemical combination
- DALTON’S ATOMIC THEORY
- SUB-ATOMIC PARTICLES
- Cathode Ray Tube & Television
- X-Ray & Roentgen
- Atomic Mass
- Isotopes of Hydrogen
- Mole and Avogadro
- Atomic Models
- Quantum Mechanic Model
- Photoelectric Effect: Einstein & Planck
- Spectrum
- Electromagnetic spectrum
- Black Bodies
- Mock Questions
Prologue
- This year’s UPSC CAPF paper contained significant number of questions science, particularly higher level chemistry (Above class7-10 NCERTs)
- In recent years, UPSC has been shifting from Class 7 to 10 Science NCERTs towards 11-12 NCERTS) – This has been evident in all 3 exams conducted by UPSC viz. CAPF, CDS and CSE-prelims.
- Therefore, with help of Venkat sir, I’m preparing selected “revision notes” out of NCERT Science Books (11, 12). Most important: can’t guarantee this project will be finished before prelim comes.
- First part of this article series deals with Class11 Chemistry NCERT Chapter 1 (Basic concepts of matter) and chapter 2 (Structure of Atom).
Matter classification

Possible MCQ: Assertion reasoning, “which of the following 2-3 statements are correct?”
SI system of measurement
- Through Metre convention in Paris (1875), this International system of units was setup.
- accordingly, each country has an institute to maintain standards of measurement
- e.g. in India it is done by National physical laboratory @Delhi.
7 Basic units in SI measurement

Possible MCQ: Macth the following
Definition of SI units

Possible MCQ: Which of the following statements correct about xyz unit?
Why Pt-IR cylinder for weight standardization?
- The Mass standard is kilogram.
- Kilogram is defined as the mass of platinum-iridium (Pt-Ir) cylinder that is stored in an airtight jar at International Bureau of Weights and Measures in Sevres, France.
- Pt-Ir was chosen for this standard because it is highly resistant to chemical attack and its mass will not change for an extremely long time.
- Assertion reasoning question possible from above factoids

Possible MCQ: Match the following prefix vs. multiple / convert “x” into “y”
DALTON’S ATOMIC THEORY
- Democritus, a Greek Philosopher (460 — 370 BC) said that matter is composed of small indivisible particles called ‘a-tomio’ (meaning — indivisible). Indian philosophers also made similar statements. But they had no proofs.
- Finally, a British Teacher John Dalton published book published ‘A New System of Chemical Philosophy’ (1808) with following points
- Matter consists of indivisible atoms.
- All the atoms of a given element have identical properties and identical mass.
- Atoms of different elements differ in mass.
- Compounds are formed when atoms of different elements combine in a fixed ratio.
- Chemical reactions involve reorganisation of atoms. These are neither created nor destroyed in a chemical reaction.
| Good | Bad |
|---|---|
his theory can explain following:
|
He couldn’t explain following
|
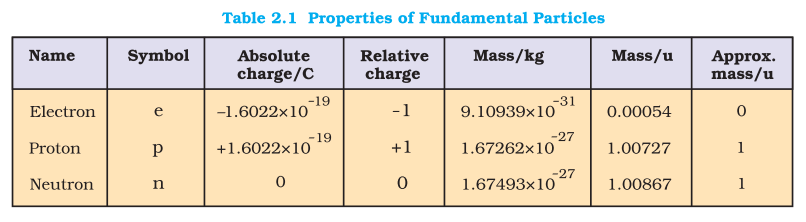
Ascending descending order of weight / charge could be asked in MCQ
| Discovery of | Was discovered by |
|---|---|
| 1.Electron | A cathode ray discharge tube – cathode ray particles – observed though fluorescent or phosphorescent – negatively charged particles, called electrons |
| 2.Proton | modified cathode ray tube – canal rays – positively charged particles |
| 3.Neutron | Chadwick (1932). he bombarded alpha-particles on beryllium thin sheet. |
Cathode Ray Tube & Television
- 1830: Michael Faraday showed that if electricity is passed through a solution of an electrolyte, matter will be liberated and deposited at the electrodes. (1830)
- 1850s: Faraday began to study electrical discharge in cathode ray tubes
- A cathode ray tube is a sealed glass tube containing two thin metal pieces (electrodes).
- Cathode rays start from cathode and move towards the anode.
- Cathode ray will travel in straight line IF there is no electrical or magnetic field,
- If there is electrical/magnetic field, cathode rays will behave like charged particles.
- Characteristics of cathode rays (electrons) do not depend upon (1) the material of electrodes (2) nature of the gas present in the cathode ray tube.
- Cathode rays themselves are not visible but their behaviour can be observed with the help of certain kind of materials (fluorescent or phosphorescent) which glow when hit by them.
- We can observe electrical discharge through the gases only at very low pressures and at very high voltages.
- Television picture tubes are cathode ray tubes
- Television pictures result due to fluorescence on the television screen coated with certain fluorescent or phosphorescent materials.
X-Ray & Roentgen
- Wilhalm Röentgen: strike electron to dense anode metal in Cathode ray tube => rays produced => these rays cause fluorescence in the fluorescent materials placed outside the cathode ray tubes. He called them X-Rays (1895)
- Henri Becqueral coined the term radioactivity. Marie Curie, Piere Curie, Rutherford and Fredrick Soddy worked further in this field.
| Detail | penetrating power |
characteristic |
|---|---|---|
| Alpha particles | 1 |
|
| Beta Rays | 100 x alpha | negatively charged particles similar to electrons |
| Gamma-Rays | 1000 x alpha |
|
Atomic Mass
- Greek word ‘stoichiometry’ =stoicheion (meaning element) + metron (meaning measure).
- Stoichiometry calculation of masses and volumes of reactants and products of a chemical reaction.
- 19th Century scientists assigned mass of “1” to Hydrogen. (no units, only number). All the remaining elements were given mass number relative to Hydrogen.
- 1961: Carbon – 12 isotop is assigned a mass of exactly 12 atomic mass unit (amu) and masses of all other atoms are given relative to this standard.
- Thus, One atomic mass unit (AMU) = one- twelfth the mass of one carbon – 12 atom.
- Today, ‘amu’ has been replaced by ‘u’ which is known as unified mass.
- Today, we have sophisticated techniques e.g., mass spectrometry for determining the atomic masses fairly accurately.
| Atomic number (Z) | Mass Number (A) |
|---|---|
|
|
|
|
|
Isobars= same “A” but different “Z” |
| Average Atomic Mass |
|
| Formula Mass | e.g. formula mass of sodium chloride = atomic mass of sodium + atomic mass of chlorine |
| Isotop | proton | neutron | rarity |
|---|---|---|---|
| protium | 1 | 0 | 99.985% hydrogen is like this |
| deuterium | 1 | 1 | 0.015% |
| tritium | 1 | 2 | trace amount in earth |
- Similarly Carbon has 3 isotopes, chlorine has 2 isotopes
- An Element’s chemical property depends on no. of electrons, and not much on neutrons. Therefore, Isotopes show same chemical behaviour. (can be asked for assertion-reasoning)
Mole and Avogadro
- One mole = Amount of a substance that contains as many particles as there are atoms in exactly 12 g (or 0.012 kg) of the Carbon 12 isotope.
- 1 mol is also known as ‘Avogadro’ constant, in honour of Amedeo Avogadro. It equals to 6.022×1023 atoms
Atomic Models
| 1.J.J.THOMSON,Britain |
|
| 2.RUTHERFORD’S NUCLEAR MODEL |
|
| 3.NEILS BOHR, Denmark |
|
| 4.Wolfgang Pauli, Austria |
|
| 5.Millikan |
|
Quantum Mechanic Model
- Classical atomic models ignore dual behavior of particles.
- Just like radiation, particles also have dual properties i.e. Wave like properties and particle like properties (French physicist, de Broglie in 1924).
- This is known as “Quantum mechanics”- Erwin Schrödinger – Nobel winner Austrian physicist was the front runner of this theoretical science.
- We can’t find the exact position and exact momentum (or velocity) of an electron at the same time, because electron and other similar particles don’t have definite paths or trajectories of electrons and other similar particles- This is Heisenberg’s Uncertainty Principle.
- Heisenberg was a German who shared Nobel with Schrödinger in Physics. He researched atomic bomb for Germany during WW2
- Electrons wave-like properties are utilized in electron microscope, it can give a magnification of about 15 million times.
Photoelectric Effect: Einstein & Planck
German physicist Max Planck observed that:
- When Light strikes surface = electrons ejected without any time lag.
- How many electrons ejected? Ans. Proportional to light’s brightness.
- How much is the kinetic energy of these ejected electrons? Ans. NOT in proportion of light’s brightness.
- If red light shined on potassium for hours but no photoelectrons are ejected.
- But even if a very weak yellow light shines on the potassium metal, the photoelectric effect is observed.
- German born American physicist Albert Einstein was able to explain this Photoelectric effect using Planck’s quantum theory of electromagnetic radiation.
- Einstein said light shining = shooting photon particle beam =collision with electrons=electrons ejected.
- Brighter light = more protons = more electrons ejected.
- He Won Nobel Prize in Physics in 1921 for his explanation of the photoelectric effect.
Spectrum
- Speed of light depends upon the nature of the medium through which it passes.
- As a result, the beam of light is deviated or refracted from its original path as it passes from one medium to another.
- The light of red colour which has longest wavelength is deviated the least while the violet light, which has shortest wavelength is deviated the most.
- Examples of continuous spectrum: (1) White light spectrum (2) rainbow. Because they have all colors from violet to red.
- The study of emission or absorption spectra is referred to as spectroscopy

Possible MCQ: ascending descending order of rays depending on thier frequencies / their utility
Electromagnetic spectrum
- Water wave and sound wave need medium. They can’t move in vaccum
- Electromagnetic waves do not require medium and can move in vacuum.
- There are many types of electromagnetic radiation depending on their wavelength (or frequency).
- Collectively, they’re called electromagnetic spectrum
| Wavelength |
|
|---|---|
| Frequency |
|
Black Bodies
Wave nature of Electromagnetic radiation can explain following:
| Diffraction | it is the bending of wave around an obstacle |
| Interference | it is the combination of two waves of the same or different frequencies to give a wave |
- But above things can’t explain a black body radiation. Later Max Planck explained it in following manner:
- When solids are heated they emit radiation over a wide range of wavelengths.
- Heating Iron rod = dull red (low frequency) => more heating => bright red color (higher frequency)=>more heating=> white=>Blue…frequency keeps on increasing, wavelength keeps on decreasing.
- Black body is the ideal body that emits and absorbs all frequencies.
- The radiation emitted by such a body is called black body radiation.
- Black Body’s radiation depends only on its temperature….At a given temperature, intensity of radiation emitted increases with decrease of wavelength, reaches a maximum value at a given wavelength and then starts decreasing with further decrease of wavelength (something like a bell curve).
- Planck suggested that atoms and molecules could emit (or absorb) energy only in discrete quantities and not in a continuous manner. This smallest energy quantity is “quantum”.
Mug up following drugs because given in class11 Chemistry NCERT
| Cisplatin, Taxol | Cancer |
| AZT: Azidothymidine | AIDS |
Mock Questions
Following type of MCQs can be framed:
- Whatever “cause-consequence” or “x because of y” type of information is given in this note, it can be utilized for assertion reasoning questions e.g. (1) why Pt-IR used in weight std. (2) how cathode-ray works in TV
- Match the following (1) scientist vs. principle. (2) basic physical quantity vs name of the SI unit (3) range of electromagnetic spectrum vs. utility of those rays (4) alpha, beta, gamma and x-rays vs their properties (similar qs. asked in CAPF) (5) isotopes of hydrogen vs. no. of neutrons present in them
- You can be given a term, and asked to identify the “factor responsible or variable attached.” example (1) Black body radiation depends on which of the following factors? (2) Cathode ray’s travel path depends on which of the following factors?
- 2 or 3 statements about Biography-contribution of Einstein- then you’re asked to identify the right or the wrong ones.
- Which of the following statements are correct about (1) Atomic mass / number (2) isotope vs. isobar (3) mass vs. weight and so on.

![[Science WMD] ISRO POEM, NASA CAPSTONE, GEMCOVAC-19, Doctors Day, Weekly Mrunal Digest from Jun week4-2022](https://mrunal.org/wp-content/uploads/2022/07/isro-poem-scaled-500x383.jpg)
![[Revision] Agriculture Chemistry: Mineral Nutrition, Plant Growth Regulators, Ethylene, Hydroponics, Photoperiodism, Vernalisation](https://mrunal.org/wp-content/uploads/2015/08/c-nitro-cycle-500x383.jpg)
Mrunal ji… Sir ji… HRD sections ok delay na karen… Please.
Perfect,..thanku so much mrunal team
plz change proton to photon in paragraph Photoelectric Effect
Sir i want total explnation of exam in Ssc crpf gd plzz
sir if not all then please try to cover biology part before pre.
Hello Mrunal sir and Venkat sir,
Thank you so much for wonderful article!!!
thanks,
swaroop
Hello Mrunal,
This is really really helpful..Please try to complete this chemistry potion before prelims. It will be highly valuable….
Regards,
Nandita.
Thanks for this and please continue this type of articles and consolidated articles on other static or selective important current topics. No one does this better.
thanks a lot sir i have clear all my doubts of eco and geo i have watched both subjects video u have saved a lot of time for students like us who are self learner i am going to give prelims 2015 exam as i am an mba student at jalandhar not having so much time just watched these videos clearly help me to cover my syllabusfor prelims sir i have opted for public administration guide me some god books regarding that.thanks a looooooooooooooooooooooooot for your contribution towards aspirants of ias. all the best to all for ias 2015
thanks a lot sir!!
Its too good .
Hii
please